In 1992, shortly after completing my PhD at the University of Vienna, Horst Felbeck (Scripps Institution of Oceanography) invited me to my first deep-sea hydrothermal vent cruise. Since then, I have joined 16 cruises, dove 13 times with HOV Alvin and worked with ROVs SuBastian, Jason and ROPOS. I have spent more than a year on R/V Atlantis and would like to thank the crew very much for their hospitality. I am extremely grateful to the following colleagues for inviting me: Craig Cary, Colleen Cavanaugh, Jim Childress, Horst Felbeck, Charles R. Fisher, Pete Girguis, Samantha Joye, Lauren Mullineaux, Scott Nooner, Kathlyn Scott, Stefan Sievert, Andreas Thurnherr.

This picture I took on my very first dive with an analogue camera through the window of Alvin in 1992. Photographs: Monika Bright
A Post Doc position in the lab of Charles R. Fisher at the Pennsylvania State University, USA in 1996, and fundings from the FWF Charlotte Bühler Program and a FWF project “Triphasic life cycle in vestimentiferans” between 1997-2003 lead to several papers on the trophosome, the organ that houses the thiotrophic symbiont Candidatus Endoriftia persephone and the nutritional interaction between symbiont and host using 14C bicarbonate pulse – chase experiments and tissue autoradiography.
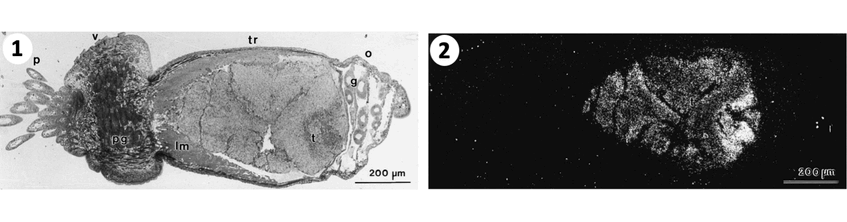
1. Light field micrograph of small tubeworm section with the body regions from to left to right: p plume, v vestimentum, tr trunk, and o ophisthosome. 2. The trunk contains the tr trophosome with the symbionts also visible due to silver grains in dark field micrograph. These grains show high carbon fixation and incorporation or organic carbon in symbionts. Photographs: Monika Bright
The next FWF funded project “Triphasic life cycle in vestimentiferans” (1999-2003) led finally to the discovery of the transmission mode. Because tubeworm larvae like to settle in cracks and crevices of the basalt but are very small and difficult to sample we designed Tubeworm Artificial Settlement Cubes (TASCs). Our first deployment was in 2001. Tubeworm babies settled in the grooves of the cube that could be taken apart and collected. This design made it finally possible to elucidate the horizontal transmission mode in tubeworms.
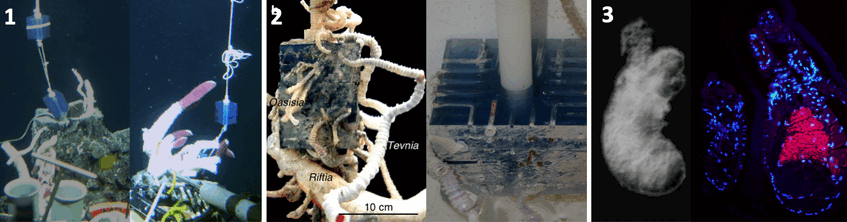
1. Deployment in 2001 (left) and recovery in 2002 (right) © WHOI 2. Settlement of three tubeworm species Riftia pachyptila, Tevnia jerichonana, and Oasisia alvinae on TASC (left side) deployed for one year at a deep-sea vent on the East Pacific Rise. Photograph: Andrea Nussbaumer 3. Small tubeworm baby without symbionts (left). Photograph: Monika Bright. Using serial sections of larvae and small juveniles and applying fluorescence in situ hybridization and transmission electron microscopy we found that the symbiont infects the host through the skin and migrates through several tissue layers into the mesoderm surrounding the gut to develop in the trophosome. FISH micrograph from section of aposymbiotic larvae and symbiotic juvenile with pink symbionts in small developing trophosome (right). Photograph: Andrea Nussbaumer
To better understand the relatives of Riftia we received funding for a further FWF project “Trophosome evolution in siboglinids” (2007-2012) to study the trophosome of Sclerolinum and Osedax.
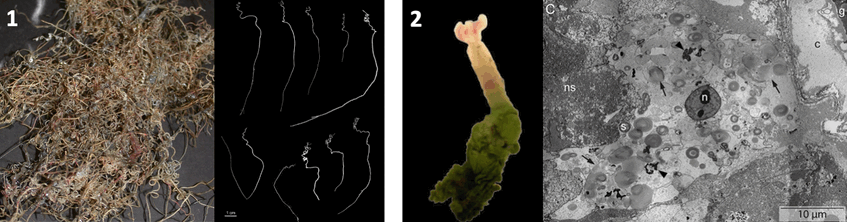
1. Sclerolinum from hydrocarbon seeps, photograph: Irmgard Eichinger and 2. Oxedax from whale falls with TEM micrograph of trophosome, photograph: Sigrid Katz
The ITN Project “Symbiomics” (2011-2014) funded the PhD of Julia Klose. After elucidating how Endoriftia enters the larval host, we were curious to find out how the symbiont escapes the host upon host death. Therefore, we designed experiments with Symbiont Recruitment Plates SRPs in high pressure flow through vessels to simulate host dead under vent and deep-sea conditions. It tuned out that Endoriftia is capable of leaving the dead trophosome tissue. They can settle on cover slips and even proliferate, not only under warm vent conditions but also under cold deep-sea condition lacking sulfide.
As theoretically predicted and found in many symbioses with horizontally transmitted symbionts, also the Endoriftia population is polyclonal. With high coverage metagenomics and multilocus sequence typing we showed that each host houses a dominant strain and several low-frequency variants. The free-living populations are more variable that the host-associated populations.
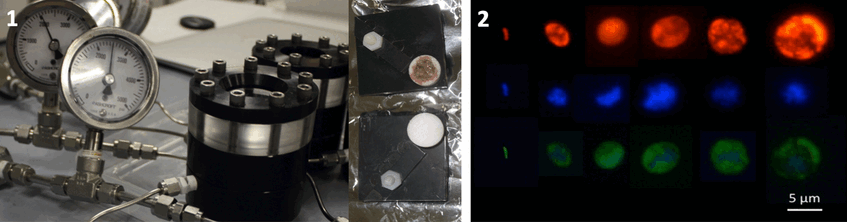
1. High pressure vessels (left) and SRP with trophosome in little porous container for symbionts to escape and settle on tiny cover slips (right). Photographs: Monika Bright (left), Julia Klose (right)
2. FISH micrographs of free-living Endoriftia (red EUBmix probe, blue DAPI, green Endoriftia specific probe). Photograph: Julia Klose
In our current FWF project “Endoriftia response to host-associated and free-living life style” (2018-2022) we aim to unravel how the symbionts manage to deal with various conditions in the host and how they leave the dead host and survive in free-living in deep-sea environments. Gene expression patterns in the symbiont from live hosts exposed to different chemical concentrations, from dead hosts, and from free-living hosts in the seawater as well as colonizing surfaces will inform about the physiology of the symbiont. Further we plan to investigate whether the symbiont can live heterotrophically, especially under environmental conditions that do not allow chemosynthesis. Therefore, a suite of experiments was carried out in high-pressure aquaria during two research cruises on the R/V Atlantis with the submersible Alvin and on the R/V Falkor with the ROV SuBastian to the Pacific Ocean. This proposed study will enhance our understanding on the evolution of this exceptional partnership.

Samples for this project come from one EPR cruise with R/V Atlantis and Alvin in 2016 © WHOI

More samples came for one Guymas Basin cruise with R/V Falkor and ROV SuBastian in 2019 © SOI
We published the genome of Riftia pachyptila and the closed genome of Endoriftia persephone in 2022. The host genome shows signs of reductive evolution. Expanded gene families reflect evolutionary adaptations to the vent environment and endosymbiosis. Despite lacking a mouth and and gut as adult, the developmental gene repertoire is extremely conserved. The trophosome is not just an organ that houses the endosymbionts it is functionally replacing the gut, stores excretory products and has hematopoietic function.
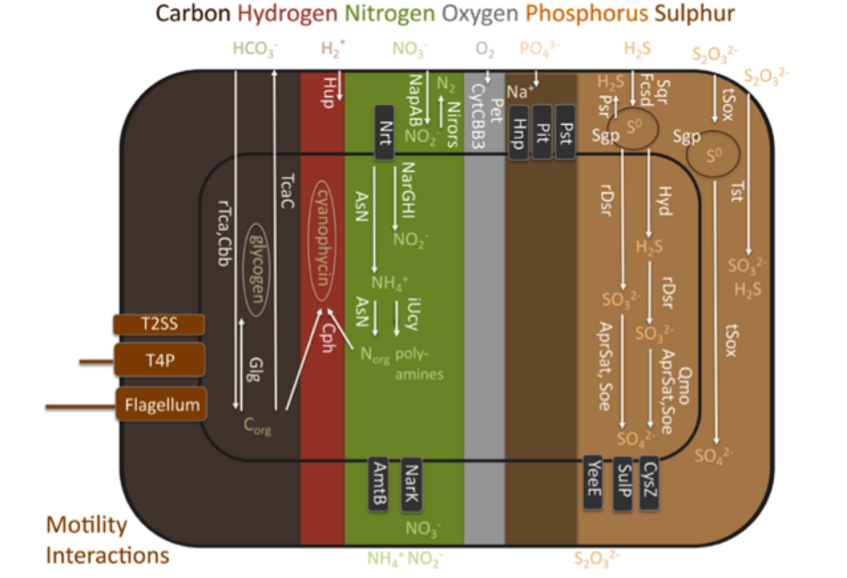
Functional traits found in the genome of Candidatus Endoriftia persephone.
AmtB, ammonium transporter; AprSat, sulphate adenylyltransferase and adenylylsulphate reductase sulphite oxidation; AsN, assimilatory nitrate reduction; Cbb, Calvin-Benson-Basham cycle; Cph, cyanophycin biosynthesis; CysZ, sulphate transporter; CytCBB3, cytochrome cbb3 based oxygen respiration; Fcsd, flavocytochrome c sulphide dehydrogenase sulphide oxidation; Glg, glycogen biosynthesis; Hnp, high affinity sodium- phosphate symporter; Hup*, putative hydrogen oxidation (Mitchell and coauthors found uptake of hydrogen apparently does not serve to fuel carbon fixation, neither was hydrogenase activity measured); Hyd, sulhydrogenase elemental sulphur oxidation; iUcy, incomplete urea cycle lacking last step arginase gene; NapAB, periplasmic nitrate reduction to nitrite; NarGHI, cytoplasmic nitrate reduction to nitrite; NarK, nitrate-nitrite antiporter; Nirors, sequential oxidation from nitrate to dinitrogen by nirS, norBC and nosZ as final steps of the denitrification process; Nrt, nitrate transporter; Pet, cytochrome bc1 complex mediated electron transport chain; Pit, low affinity phosphate transporter; Psr, polysulphide reductase elemental sulphur reduction; Pst, high affinity phosphate transporter; Qmo, quinone-interacting membrane- bound oxidoreductase mediated sulphite oxidation; rDsr, reverse dissimilatory sulphate reductase mediated sulphur oxidation; rTca, reductive TCA cycle; Sgp, sulphur globule proteins; Soe, sulphite-oxidation enzyme sulphite oxidation; Sqr, sulphide:quinone oxydoreductase sulphide oxidation; SulP, sulphate permease; T2SS, type II secretion system; T4P, type IV pilus; TcaC, TCA cycle; tSox, truncated Sox mediated sulphur oxidation; Tst, thiosulphate disproportionation; YeeE, thiosulphate transporter
