In 1831 Hemprich and Ehrenberg described the colonial ciliate Zoothamnium niveum. More than 150 years later Jörg Ott found a very large ciliate in the mangroves of the Belize. The white color turned out to be a coat of sulfur bacteria.
During two post-doctoral positions at the University of Tübingen, Germany and the Rosenstiel School of Marine and Atmospheric Science, University of Miami, funded by an FWF Erwin Schrödinger-Postdoctoral Fellowship and an Emil-Boral-Postdoctoral Fellowship between 1993-1995 we re-described the host species, for which I got awarded the John O. Corliss Ciliate Systematics Award in 1997.
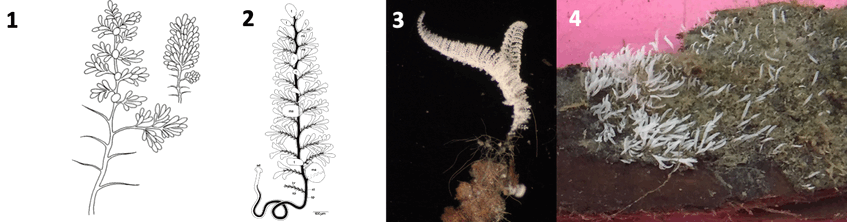
1. Original drawing of expanded and contracted colony by Hemprich F. W. and Ehrenberg C. G. (1829, 1831) Symbolae physicae. Evertebrata. I. Phyotozoa. Abhandlungen der Akademie der Wissenschaften zu Berlin
2. Drawing: Monika Bright
3. Colony collected from shallow wood fall in the Northern Adriatic Sea. Photograph: Monika Bright
4. Culture on wood from the Northern Adriatic Sea in 2020. Photograph: Monika Bright
In the FWF project "Life cycle and interactions of a thiotrophic symbiosis" (2003-2007), we characterized the symbiont Candidatus Thiobius zoothamnicola.
Most importantly, Christian Rinke was able to cultivate the ciliate. This is, to our knowledge, still the only thiotrophic symbioses that can be cultured over several host generations. We developed tiny flow-through aquaria to keep oxygen and hydrogen sulfide concentrations stable. Highest growth rates were found under oxic conditions supplemented with low sulfide concentrations. Interestingly, the host also grew without sulfide but reached only small sizes.
At that time, we did not understand how important this finding was.
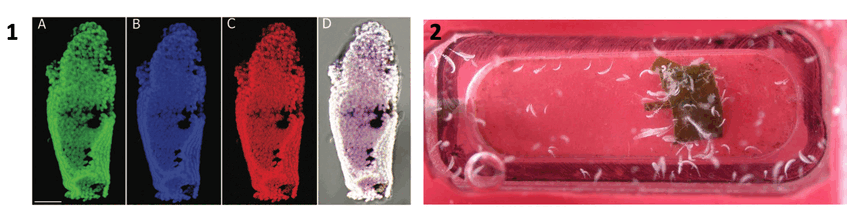
1. Fluorescence in situ hybridization micrograph showing section of ciliate with Cand. Thiobios zoothamnicoli (DAPI in blue, symbiont probe in green, EUBmix and Archaea probe in red, and overlay with all three colors showing the symbionts in white. 2. Culture in tiny flow-through aquaria. Photographs: Christian Rinke
In the next FWF project “Thiotrophic mutualism – cooperation goes empirical” (2012-2016) we relocated our field research to the Marine Biology Station in Piran. We are very grateful for their hospitality and continuous support.
We finally unraveled why this ciliate did not die under oxic conditions. We discovered a spectacular polyphenism – two distinct phenotypic growth forms in the ciliate, a symbiotic one and an aposymbiotic one. We showed in a suite of experiments that polyphenism in this mutualism is triggered by chemical conditions and induced by the symbiont’s presence on the dispersing swarmer.
In a follow-up study we exposed the colonies to oxic seawater mimicking waning of sulfide to study the fate of both partners. We found that colonies released some initially present but also newly produced macrozooids until death, but in fewer numbers than when exposed to sulfide. Below you see the mortality of colonies (left) and swarmers (right) with and without sulfide. The symbionts on the colonies proliferated less without sulfide, and became larger and more rod-shaped than symbionts from freshly collected colonies that were exposed to sulfide and oxygen. The symbiotic monolayer was severely disturbed by growth of other microbes and loss of symbionts. We conclude that the response of both partners to the termination of sulfide emission was remarkably quick. The development and the release of swarmers continued until host died and thus this behavior contributed to the continuation of the association.
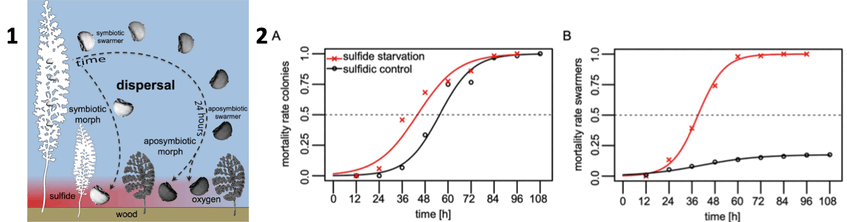
1. Proposed development of symbiotic morphs from symbiotic swarmers released from colony and short migration through oxic water prior to settlement on sulphide-emitting wood surface. In contrast, long migration of swarmers in oxic seawater leads to loss of symbionts and development of aposymbiotic morphs under both oxic and sulphidic conditions. Drawing: Monika Bright. 2. The sulfide starvation experiment is shown in red and the sulfidic control in black. (A) Binomial Generalized Linear Model of the mortality of the colonies given as the proportion of dead colonies in relation to the total number of colonies. LT50 is indicated as the point of intersection with the dashed line. Pictures: Salvador Espada-Hinojosa
We investigated this nutritional relationship in pulse-chase experiments using 14C bicarbonate tissue autoradiography, 13C bicarbonate NanoSIMS and correlative TEM. We found that this facultative host is milking and farming the symbiont. Nourishment is accomplished by uptake of released fixed organic carbon from the symbiont but also through digestion of symbionts and a few free-living microbes.
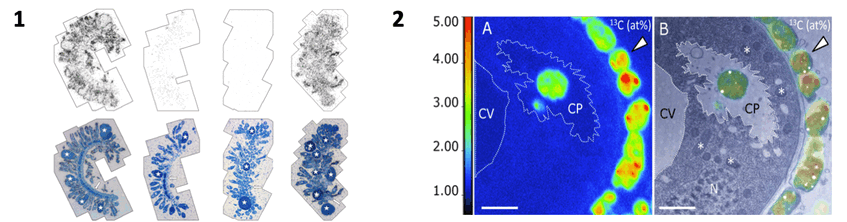
1. Tissue autoradiography – silver grains overlain by semi-thin section differ according to pulse treatment with 14C bicarbonate in seawater from left to right with 1) sulfide and oxygen, 2) oxygen but no sulfide, 3) 24 h oxygen prior pulse in oxygen 4) sulfide and oxygen and then chase with oxygen but without sulfide. Pictures: Jean-Marie Volland
2. NanoSIMS/TEM correlative images after a sulfidic pulse in the presence of 13C bicarbonate. Highly labelled symbionts are visible outside of the host (arrowhead) and one symbiont is located in cytopharnx (CP) next to contractile vacuole (CV). Pictures: Jean-Marie Volland
In our current FWF project “Environmental impact on giant ciliate mutualism” (2019-2023) we aim to unravel the mechanisms that have evolved in this mutualism to deal with the notoriously instable environment over short, annual, and longtime scales.

One of our collections sites – the Canal of Sv. Jernej close to Piran. Photograph: Monika Bright

A little graveyard of wooden boats in the Canal of Sv. Jernej that we visited for several years. Unfortunately, they were taken out last year so one of our great collections sites is gone now. Photograph: Monika Bright
